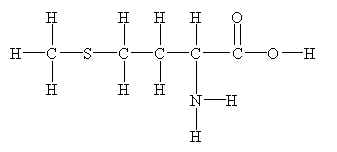
Metiyonin saçın yapım maddesi olan keratinin ön maddesidir.Amino asittir,L-Sistinle adlı diğer bir aminoasitle birlikte keratin yapımını sağlar.Keratin saçın yapım maddesidir ve eksikliğinde ciddi saç sorunları görülür.
Metionin gıdalarla alınmaktadır ancak dışarıdan ayrıca alınması keratin üretim hızını artırır ve saç kalitesini olumlu yönde etkiler.Saç sağlığıyla ilgili üretilen tüm ürünlerde çeşitli dozlarda metionin bulunmaktadır.
Aşağıda okuyacağınız bilimsel çalışma metioninin saç beyazlamasını engellediğini kanıtlamaktadır.
Senile hair graying: H2O2-mediated oxidative stress affects human hair color by blunting methionine sulfoxide repair
J. M. Wood * † , 1 , H. Decker ‡ , H. Hartmann ‡ , B. Chavan * , H. Rokos * † , J. D. Spencer * † , S. Hasse * † , M. J. Thornton * , M. Shalbaf * , R. Paus § ‖ and K. U. Schallreuter * , 2
2 Correspondence: Clinical and Experimental Dermatology/Department of Biomedical Sciences, University of Bradford, Bradford, BD7 1DP, West Yorkshire, UK.
Abstract
Senile graying of human hair has been the subject of intense research since ancient times. Reactive oxygen species have been implicated in hair follicle melanocyte apoptosis and DNA damage. Here we show for the first time by FT-Raman spectroscopy in vivo that human gray/white scalp hair shafts accumulate hydrogen peroxide (H2O2) in millimolar concentrations. Moreover, we demonstrate almost absent catalase and methionine sulfoxide reductase A and B protein expression via immunofluorescence and Western blot in association with a functional loss of methionine sulfoxide (Met-S=O) repair in the entire gray hair follicle. Accordingly, Met-S=O formation of Met residues, including Met 374 in the active site of tyrosinase, the key enzyme in melanogenesis, limits enzyme functionality, as evidenced by FT-Raman spectroscopy, computer simulation, and enzyme kinetics, which leads to gradual loss of hair color. Notably, under in vitro conditions, Met oxidation can be prevented by L-methionine. In summary, our data feed the long-voiced, but insufficiently proven, concept of H2O2-induced oxidative damage in the entire human hair follicle, inclusive of the hair shaft, as a key element in senile hair graying, which does not exclusively affect follicle melanocytes. This new insight could open new strategies for intervention and reversal of the hair graying process.—Wood, J. M., Decker, H., Hartmann, H., Chavan, B., Rokos, H., Spencer, J. D., Hasse, S., Thornton, M. J., Shalbaf, M., Paus, R., Schallreuter, K. U. Senile hair graying: H2O2-mediated oxidative stress affects human hair color by blunting methionine sulfoxide repair.
SKIN AND HAIR COLOR HAVE BEEN at the center of attention of humans since ancient times. Graying of the hair (canities) has major psychosocial and socioeconomic implications, since it is often regarded as a sign of rapidly progressing old age, ill health, and bodily decline—especially in today’s world, where humans are confronted with increasing pressure to stay “forever young and vital.” Hence, senile and premature graying has long attracted researchers and industry alike with scientific as well as commercial targets. Yet, apart from various hair dyes of varying efficacy and duration, fully satisfactory solutions for the graying problem remain to be brought to market. A key reason still not addressed is that the underlying molecular and cellular mechanisms of graying remain under debate (1⇓ 2⇓ 3)⇓ .
So far, the biological process of hair graying has been attributed to the loss of the pigment-forming melanocytes from the aging hair follicle, including the bulb and the outer root sheath (1⇓ , 2⇓ , 4⇓ 5⇓ 6⇓ 7⇓ 8⇓ 9⇓ 10⇓ 11⇓ 12)⇓ . In this context, it is of interest that activity of hair bulb melanocytes is under cyclic control (1⇓ , 13)⇓ . Both melanogenesis and hair shaft production take place in the anagen phase of the hair cycle. Toward the end of this phase, the pigment-forming melanocytes retract their dendrites and stop melanogenesis, which is followed by apoptosis-driven regression in the catagen phase and the final resting telogen phase. Theories for the gradual loss of pigmentation include exhaustion of enzymes involved in melanogenesis, impaired DNA repair, loss of telomerase, antioxidant mechanisms, and antiapoptotic signals including the loss of Bcl-2 and decreased stem cell factor (2⇓ , 4⇓ , 6⇓ , 14⇓ 15⇓ 16⇓ 17)⇓ . Vacuolation in hair bulb melanocytes has been ascribed to oxidative stress (18)⇓ . By analogy with the free radical theory of aging, recently a “free radical theory of graying” has been proposed (4)⇓ . Generation of H2O2 has been attributed to its intrinsic release from the melanogenesis pathway, ultimately yielding apoptosis of hair follicle melanocytes (HFMs) and DNA damage (4)⇓ . Decline of melanogenesis is associated with loss of tyrosinase (EC 1.14.18.1) activity (2⇓ , 14⇓ , 19⇓ , 20)⇓ , which affects in turn the rate-limiting step in melanogenesis. Here it is of interest that low H2O2 concentrations (<0.3×10−3 M) increase tyrosinase activity, while high concentrations (10−3 M) irreversibly deactivate the enzyme (21⇓ , 22)⇓ . Abundant evidence indicates that many proteins and peptides, including the H2O2-reducing enzyme catalase (EC 1.11.1.6) as well as the two repair mechanisms for free and bound methionine sulfoxide (Met-S=O), methionine sulfoxide reductases A and B (MSRA and MSRB; EC 1.8.4.6 MSRA&B), are structurally damaged and functionally altered by H2O2-mediated oxidation. This finding is based on the presence of methionine (Met), cysteine (Cys), and tryptophan (Trp) residues in their protein sequence (23⇓ 24⇓ 25⇓ 26⇓ 27)⇓ . Bearing all these facts in mind, it was tempting to readdress the potential of H2O2 in senile hair graying. By analogy, we turned to vitiligo, a depigmentation disorder, as this model could hold lessons for a better understanding of the graying process. In this disease, H2O2-mediated oxidative stress plays a central role in the loss of inherited skin color due to generation and accumulation of millimolar H2O2 concentrations in the epidermal compartment, as documented in vivo by Fourier transform Raman spectroscopy (FT-Raman spectroscopy) (11⇓ , 28)⇓ . Given that hair color is exclusively produced in the pigmentary unit of the anagen III-VI hair follicle (2⇓ , 14⇓ , 19)⇓ , we postulated that accumulation of H2O2 could be a contributing factor in the hair graying process due to deactivation of catalase as well as of MSRA and MSRB, which in turn would affect the formation of hair color via tyrosinase in HFMs (11⇓ , 25⇓ , 26⇓ , 28)⇓ .
This idea was supported by the fact that precursor tyrosinase contains 17 Cys, 14 Trp, and 15 Met residues in its sequence, according to the Swiss-PROT database (http://www.expasy.org), while in mature tyrosinase (19–529), 16 Cys, 13 Trp, and 14 Met residues are present, which are all potentially susceptible to oxidation. Additional support for this hypothesis arrives from a 3-dimensional model of the mammalian tyrosinase active site (29⇓ , 30)⇓ . Homology modeling of the murine enzyme and delineation of key amino acids in the active site of this enzyme with the help of site-directed mutation analyses revealed that Met 374 was essential for tyrosinase activity, because mutation of this residue to Gly 374 caused a 93% loss of enzyme activity (29)⇓ . Here it is noteworthy that 85% of the primary sequences in mouse and human tyrosinases are conserved, with 92% structural homology. Further support for the critical importance of Met 374 stems from studies with human type 1 oculocutaneous albinism (OCAI), where the orientation of this Met residue is affected by the Ser 380 to Pro 380 polymorphism (31⇓ , 32)⇓ . Here this structural change destabilizes Met 374 and disrupts the coordination of His residues to the Cu-(B) active site of tyrosinase, leading to the loss of enzyme activity (33)⇓ .
To probe our hypothesis, we applied in vivo FT-Raman spectroscopy, and we show for the first time the presence of millimolar H2O2 concentrations and H2O2 oxidation products in the native senile human gray/white anagen hair shaft. We also show for the first time the presence and function of Met-S=O repair capacity by the enzymes MSRA and MSRB in situ and on the functional level in human isolated anagen hair follicles and in isolated different human hair follicle cells. Notably, protein expression as well as its function is nearly completely abrogated in the gray hair follicle. Our results are in agreement with the near absence of H2O2-reducing capacity by catalase, which affects the entire gray hair follicle. In addition, we show for the first time formation of Met-S=O in H2O2-oxidized tyrosinase by in vitro FT-Raman spectroscopy, and we prove loss of enzyme function after oxidation. These results are supported in cell extracts from human epidermal melanocytes (EMCs) and by enzyme kinetics. Computer modeling of the tertiary structure of H2O2-oxidized tyrosinase reveals enzyme deactivation due to Met 374 instability in the enzyme active site. Notably, H2O2-mediated oxidation of tyrosinase can be prevented through free L-methionine, which in turn acts as an H2O2 scavenger via formation of Met-S=O.
MATERIALS AND METHODS
All studies on human material were approved by the local ethics committees and were in agreement with the Helsinki declaration.
Cell culture of dermal papilla (DP) cells, dermal fibroblasts (DFs), dermal sheath (DS) cells, and EMCs
Hair follicle cell cultures were established from face-lift surgery (n=2 female) and healthy donors (n=3 male) after signed consent. The study was in agreement with the principle of the Helsinki declaration and was approved by the local ethics committees. To establish primary cultures of DFs, the skin samples were dissected through the dermis; divided into 3- × 3-mm pieces; and transferred to a 25-cm2 tissue-culture flask (Dow Corning, Corning, NY, USA). The samples were then cultured in DMEM (Life Technologies, Inc., Paisley, Scotland) supplemented with glutamine (10 mM), penicillin (100 U/ml), streptomycin (100 μg/ml), and amphotericin B (250 μg/ml), with 20% fetal calf serum (FCS) (Sigma, Poole, Dorset, UK) at 37°C in 5% CO2 in air. To establish primary cultures of DP and DS cells, terminal hair follicles were dissected individually and cleaned from fat using two 7.5-gauge needles. The upper part of the hair follicle was removed. The hair shaft was removed from the lower part of the hair follicle by applying gentle pressure to the base, leaving the DS and the DP. To isolate the DP, a slit was made in the DS, which was inverted by itself to expose the DP on its stalk at the base of the follicle. The DP was separated from the DS by dissecting through its stalk and transferred to a 35-mm dish containing 2 ml of MEM supplemented with 20% human serum. Once the DP cells were isolated, the lower part of the DS was transferred to a separate 35-mm dish containing 2 ml DMEM supplemented with 20% human serum. A scratch was made through the tissue to aid attachment to the dish and encourage growth and migration of the DP or DS cells. Approximately 6 DP or DS cells were cultured in each dish and clustered to encourage cell migration. The dishes were left undisturbed for 7 d. After this period, the growth medium was refreshed, and the explants were checked for cell migration. This step was repeated every 2–3 d until sufficient growth had occurred to allow passaging of the cells with resuspension into a T25 flask. Once explant cultures were established, the medium was supplemented with 10% FCS. EMCs were cultured from face-lift surgery (n=2) following the method of Pittelkow and Shipley, as described in detail elsewhere (34)⇓ . Human HFMs were a generous gift from D. J. Tobin (University of Bradford, Bradford, UK).
Preparation of human anagen hair follicle
Human hair follicles were isolated from normal human scalp skin obtained from routine face-lift surgery after patient consent (n=6; 4 female, 2 male). Anagen VI hair follicles were isolated using a microdissection microscope according to a modified method of Philpott (35)⇓ . Briefly, scalp skin was cut in small pieces of ∼1 cm2 in size. The dermis was separated from the subcutis, giving rise to the midlower part of anagen VI hair follicles, which were extracted with fine forceps.
Preparation of cell extracts from full hair follicles and hair follicle cells
To avoid protein denaturation, cell extracts were obtained from intact anagen hair follicles and cell cultures utilizing a minipestle, a mortar (−80°C), and fine sand. Cell pellets and hair follicles were ground in ice-cold Tris buffer (0.05 M, pH 7.5) followed by centrifugation at 7000 g for 5 min. The supernatant was collected, divided into aliquots, and stored at −80°C until further use. The protein content was determined by the Dc-protein assay (Bio-Rad, Hercules, CA, USA).
In situ immunofluorescence protein detection in the isolated human hair follicle
Freshly isolated hair follicles were embedded in OCT™ compound (Sakura, Eastbourne, UK) and kept at −80°C until further processing with the cryostat (Leica Microsystems, Milton Keynes, UK). Frozen slides with 5-μm cryosections of gray hair (n=3) and dark hair (n=3) follicles and shafts were air-dried for 1 h at room temperature, fixed in ice-cold methanol for 6 min, and blocked in 10% normal donkey serum (NDS, Jackson Immunoresearch Laboratories, Cambridge, UK) for 90 min, followed by a 5-min wash in phosphate buffered saline (PBS). MSRA was detected by using a polyclonal rabbit anti-human antibody (Autogen Bioclear, Calne, UK) diluted 1:50 in 1% NDS, followed by incubation at room temperature for 3 h. MSRB detection utilized a monoclonal mouse anti-human antibody (Autogen Bioclear) diluted 1:50 in 1% NDS and incubated overnight at 4°C. The detection of catalase utilized a monoclonal mouse anti-human antibody (Sigma) diluted 1:100 in 1% NDS and incubated overnight at 4°C. Slides were washed 4× with PBS, air-dried, and incubated for 1 h at room temperature with a fluorescent secondary antibody [fluorescein isothiocyanate (FITC)- or tetramethylrhodamine isothiocyanate (TRITC)-conjugated donkey anti-rabbit or anti-mouse at a dilution of 1:100 (Jackson Immunoresearch Laboratories)] followed by a wash 3× with PBS, air-dried, and mounted in Vectashield Mounting Medium (Vector Laboratories, Peterborough, UK) containing DAPI (4′,6-diamidino-2-phenylindole) for nuclear identification. Slides were viewed under a Leica DRMIB/E fluorescence microscope (Leica Microsystems, Wetzlar, Germany), and images were captured using a digital camera (C8484–05G; Hamamatsu Photonics UK, Welwyn Garden City, UK) coupled to a computer and evaluated with the imaging software IPLab 3.7.4 (Scanalytics, Fairfax, VA, USA).
Western blotting
Gray and brown hair follicle extracts were obtained as described above. Normal human EMC and epidermal keratinocyte extracts were used as positive controls and obtained as described previously (23⇓ , 36⇓ , 37)⇓ . Sample buffer (10% SDS, mercaptoethanol, glycerol, and 0.5 M Tris/HCl) was added to the supernatants before loading on to a 12% polyacrylamide gel for protein separation. The polyacrylamide gel was then electroblotted onto a PVDF membrane (Millipore, Billerica, MA, USA) before any nonspecific binding sites were blocked by immersing the membrane in a 0.5% gelatin (Sigma)/TBS-T buffer (20 mM Tris buffered saline with 0.047% Tween, pH 7.4) blocking solution for 2 h at room temperature. This step was followed by an overnight incubation at room temperature with the primary antibodies in 0.05% gelatin/TBS-Tween. The antibodies used were rabbit anti-MSRA (Labfrontier, Seoul, South Korea; 1:2000), rabbit anti-MSRB (Labfrontier; 1:2000), mouse anti-catalase (Sigma; 1:2000), and goat anti-actin (Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA; 1:500). Following a wash of 40 min in TBS-T buffer, the blot was further incubated for 1 h at room temperature with an anti-rabbit, mouse, or goat immunoglobulin G (IgG) peroxidase-conjugated antibody (Sigma; 1:5000). Visualization of the specific protein bands was performed using modified enhanced chemiluminescence (ECL) fixed on a film sheet (X-OMAT; Kodak, Rochester, NY, USA).
Determination of tyrosinase activity
Tyrosinase activity was based on the rate of L-dopachrome formation, measured spectrophotometrically at the optical density of 475 nm (Pharmacia, Milton Keynes, UK), when L-tyrosine was used as substrate. The reaction mixture contained 84 U of mushroom tyrosinase (Sigma) and different L-tyrosine (Sigma) concentrations ranging from 0 to 5 × 10−3 M in a final reaction volume of 1 ml potassium phosphate buffer (0.1 M, pH 7.0). The rate of reaction was determined over the linear period of 2 min. Since 1 × 10−3 M L-tyrosine produces 1 × 10−3 M H2O2 (21)⇓ , we used 3 × 10−3 M L-tyrosine (Sigma). The experiments were performed in the presence of different L-methionine concentrations (2–8×10−3 M). All experiments were performed in duplicate.
Since Met-tyrosinase is activated by L-dopa, we decided to follow L-dopachrome formation from L-dopa at 570 nm. L-Dopachrome was determined every 2 min over 15 min using a microplate reader (Dynex Technologies, Worthing, UK). To test the effect of L-methionine as an H2O2 scavenger, we tested the capacity of L-methionine to prevent H2O2-mediated oxidation. Briefly, both L-methionine and H2O2 (1:1, 5:1, 10:1) were incubated for 15 min to allow the formation of Met-S=O. Then this complex was added to the above reaction, and measurements were taken every 2 min. H2O2 scavenging was also tested in the presence of 10 × 10−3 M L-methionine where only the H2O2 concentrations were changed (0.8, 1.6, 3.3, 6.6×10−3 M). Enzyme rates were determined over the linear reaction period per minute per milligram tyrosinase.
Determination of tyrosinase activities in EMC and HFM extracts
To determine tyrosinase activity in melanocytes we used microplates. Briefly, cell extracts (100 μl) of two different primary human EMC lines (n=2) and two different pigmented HFM lines (n=2) were placed into a 96-well plate, and 11 μl L-dopa (2×10−3 M) (Sigma) was added. The formation of L-dopachrome was determined at 570 nm every 10 min over 90 min using a microplate reader (Dynex Technologies). The product formation was followed by subtracting the blank from each obtained value. The reaction rate was again determined over the linear reaction period per minute per milligram of protein. To study the effect of H2O2 on tyrosinase, enzyme activity was determined in the presence (2×10−3 M) and absence of H2O2 in EMC extracts. Experiments were performed in triplicate. Due to limitation of HFM extract, we were unable to repeat this experiment in these cells. Reaction rates were determined per minute per milligram of protein.
Determination of MSRA and MSRB enzyme activities
Complete hair follicle extracts were prepared from isolated hair follicles as described above for EMCs/HFMs and other hair follicle cells. Briefly, the reaction mixture contained 50 μl cell extract, dithiothreitol (DTT) as an electron donor (10 μl, 5×10−3 M), and [14C]methionine sulfoxide (10 μl, 5×10−3 M) (23⇓ , 36)⇓ . Reactions were incubated for 60 min at room temperature. Then 5 μl of the reaction product was applied to a TLC silica gel plate (GF 1000 μm; Merck, Darmstadt, Germany) and chromatographed in isopropanol:formic acid:water (20:1:10). L-Methionine and Met-S=O were detected by ninhydrin. Radiolabeled [14C] L-methionine spots were scraped from the TLC plates and added to 3.0 ml of scintillation fluid (Ready Safe; Beckman Coulter, Fullerton, CA, USA) and counted on the 14C channel in a Packard Tricarb Liquid Scintillation Counter (2001 TR; Packard Instruments, Meriden, CT, USA). The amount of [14C] L-methionine formed was standardized to micromoles per milligram of protein per 30 min.
In vitro and in vivo FT-Raman spectroscopy for detection of H2O2, Met-S=O, cysteic acid, 5-OH-tryptophan, and kynureneic acid in H2O2-oxidized tyrosinase and gray hair
FT-Raman spectra were acquired using a Bruker RFS 100/S spectrometer (Bruker, Karlsruhe, Germany) with a liquid-nitrogen-cooled germanium detector. Near-infrared excitation was produced by a Nd3+:YAG laser operating at 1064 nm. Each spectrum was accumulated over 17 min with 1000 scans and a resolution of 4 cm−1. Detection of H2O2 is based on the O=O stretch at 875 cm−1 (28)⇓ . The Met-S=O stretch was visualized at 1030 cm−1 accordingly (38)⇓ . Cysteine and its oxidation product cysteic acid is assigned to the SO stretch at 1040 cm−1 (39)⇓ . L-Tryptophan and its oxidation products 5-OH-tryptophan (930 cm−1) and N-formyl-kynurenine/kynurenine (1050 cm−1) were recently assigned (24)⇓ . Native and H2O2-oxidized tyrosinase (10×10−3 M) were lyophilized and measured as solids. Native human gray, completely white, and brown/black hair shafts from the scalp hair were cut into small pieces (1 mm) and analyzed by FT-Raman spectroscopy.
Computer modeling of tyrosinase
A 3-D homology model of mTy (Mus musculus, gi:6755913) and hTy (Homo sapiens, gi:401235) was reconstructed based on the crystal structure from a tyrosinase as previously reported (29)⇓ . Both mTy and hTy are 98% identical around the active site. The alignment and corrections are performed as described previously (29)⇓ . Two diastereomers of oxidized methionine require a tetrahedral coordination with an open coordination. The molecular graphics were generated with Yasara Twinset 7.12.5 (40)⇓ and rendered with Povray 3.6 (www.povray.org). For MD simulations, the Yasara 7.12.5 package was used, applying force field yasara3. For calculation, a water-filled cell was used.
RESULTS
In vivo FT-Raman spectroscopy identifies the presence of millimolar H2O2 concentrations and Met-S=O in the senile gray and white hair shaft
FT-Raman spectra show in vivo the presence of 10−3 M H2O2 concentrations in gray and completely white hair, although no H2O2 is detectable in pigmented brown hair (Fig. 1⇓ ). H2O2 assignment is based on the O=O stretch at 875 cm−1 (28)⇓ . Moreover, we demonstrate oxidation of Met residues to Met-S=O (assigned at 1030 cm−1), Trp residues to 5-OH-Trp (assigned at 930 cm−1), and N-formyl kynurenine/kynurenine (assigned at 1050 cm−1) and Cys residues to cysteic acid (assigned at 1040 cm−1) (24⇓ , 38⇓ , 39)⇓ (Fig. 1⇓ ). Based on these data, we can conclude that gray/white hair shows massive H2O2 concentrations associated with H2O2-mediated oxidation of critical amino acid residues.
Figure 1.
FT-Raman spectroscopy identifies in vivo millimolar amounts of H2O2 and its oxidation products in gray and white hair shafts. Peaks are assigned accordingly: •, H2O2 at 875 cm−1; §, phenylalanine at 1004 cm−1; ∗, Met-S=O at 1030 cm−1; #, 5-OH Trp at 930 cm−1; ▪ N-formylkynurenine/kynurenine at 1050 cm−1; ♦, cysteic acid at 1040 cm−1). Spectrum 1, brown hair; spectrum 2, gray hair; spectrum 3, white hair. Note that no evidence for H2O2 or any oxidation products was found in brown hair.
Human hair follicle cells hold the capacity for functioning Met-S=O repair
As discussed above, H2O2 oxidizes free and protein-bound Met to R- and S-Met-S=O, which are normally repaired by MSRA and MSRB, respectively (41)⇓ . However, with old age, both enzyme levels decrease, and, in the case of H2O2-mediated stress, this important mechanism is susceptible to oxidation by this ROS (36⇓ , 42)⇓ . To the best of our knowledge, so far the presence of MSRA and MSRB in the human hair follicle has not been documented. Here we show in vitro protein expression of catalase, MSRA, and MSRB proteins in human HFMs, DP cells, DS cells, and DFs by immunofluorescence and Western blot analysis in all cell types (Fig. 2A⇓ ). Determination of enzyme function of MSRA and MSRB in cell extracts from those cells reveal significantly higher activities in DP cells compared to DFs and DS cells, while enzyme activities in HFMs are similar to EMCs, as recently documented (36)⇓ (Fig. 2B⇓ ). It is interesting that DP cells have at least under in vitro conditions the highest repair capacity. This result supports the importance of a very efficient redox balance/repair within the central position of the papilla, and it needs further investigation.
Figure 2.
Human hair follicle cells hold functioning MSRA and MSRB activities. A) In vitro expression of MSRA and MSRB in human hair follicle cells. a) Scheme of the anagen hair follicle, showing location of DP, DS, DFs, and HFMs. b) Immunoreactivity expression of MSRA, MSRB, and catalase in HFMs, DP, DS, and DFs (green, FITC-labeled; red, TRITC-labeled). Expression of catalase was included as internal marker for H2O2-mediated oxidative stress (11)⇓ . c) Western blot confirms catalase, MSRA, and MSRB expression at the expected size in HFM extracts. B) MSRA and MSRB enzyme activities are present in hair follicle cells. Specific activities are shown in micromoles per milligram of protein per 30 min. DP cells, n = 3; DFs, n = 3; DS, n = 3; HFMs, n = 2; EMCs, n = 2; EKCs (epidermal keratinocytes), n = 2. Note the high expression in DP and HFMs/EMCs, underscoring the importance of Met-S=O repair in these cells. Error bars = ±SEM.
Low catalase levels in the gray human hair follicle correlate with decreased Met-S=O repair
One major degrading enzyme for H2O2 is the ubiquitous heme protein catalase. Low concentrations of the enzyme have been shown in old age, including the aging hair follicle, and in vitiligo (43⇓ 44⇓ 45)⇓ . In this context, it is noteworthy that oxidation of catalase by its own substrate causes enzyme deactivation (25⇓ , 26)⇓ . In situ protein expression of catalase, MSRA, and MSRB shows greatly reduced levels in the gray hair follicle compared to the pigmented follicle (Fig. 3A⇓ ). Western blot analysis supports the decreased in situ protein expression in the gray anagen hair follicle (Fig. 3B⇓ ). These data are substantiated further by low MSRA and MSRB enzyme activities in anagen hair follicle extracts from gray compared to brown hair follicles (Fig. 3C⇓ ). Compelling evidence in vivo and in vitro indicates that H2O2-mediated oxidative stress in the entire graying anagen hair follicle lowers both catalase and Met-S=O repair via MSRA and MSRB, feeding in turn a vicious cycle for redox dysbalance.
Figure 3.
Reduced MSRA and MSRB protein expression correlates with reduced enzyme function in the gray hair follicle. A) Reduced in situ expression of catalase, MSRA, and MSRB in the entire gray human anagen hair follicle. Decreased catalase expression is in agreement with H2O2-oxidized enzyme (11)⇓ . DAPI (blue) indicates nuclei in the corresponding hair follicle (×400). B) Western blot confirms reduced catalase, MSRA, and MSRB in human gray anagen hair follicle extracts. EMCs (EC) and epidermal keratinocytes (KC) served as positive controls (36)⇓ . C) Decreased MSRA and MSRB activities in human gray anagen hair follicle extracts. Enzyme activities were determined in 3 samples/hair color. Activities are assessed in micromoles per milligram of protein per 30 min. rMSRA/MSRB activity served as positive control. Error bars = ±SEM.
In vitro FT-Raman spectroscopy confirms formation of Met-S=O formation in tyrosinase after H2O2-mediated oxidation
We next turned our interest to tyrosinase, which is the key enzyme for melanogenesis. Considering that this enzyme contains several targets for H2O2-mediated oxidation in its sequence, including a crucial methionine in position 374 in the active site, we decided to utilize again FT-Raman spectroscopy to follow the oxidation products. The results identified the presence of Met-S=O as a fingerprint of oxidized Met residues, cysteic acid from Cys, and 5-OH-Trp/N-formylkynurenine/kynurenine from Trp residues in the oxidized enzyme (Fig. 4A⇓ ). The oxidized enzyme has no activity (Fig. 4 ).
Figure 4.
Free L-methionine acts as an effective H2O2 scavenger. A) In vitro FT-Raman spectroscopy confirms the presence of Met-S=O in oxidized mushroom tyrosinase. Spectrum shows oxidation of methionine residues to Met-S=O (∗), tryptophan to 5-OH-tryptophan (#), and cysteine to cysteic acid (♦) with the phenylalanine peak (§). B) H2O2-oxidized tyrosinase has no activity. Enzyme activity was determined in mushroom tyrosinase following the L-dopachrome formation at 475 nm over 10 min in the absence of H2O2 (♦) and in the presence of laser-induced H2O2 (▴). This result supports enzyme deactivation due to oxidation of Met 374 in the active site. C) L-Met prevents H2O2-mediated oxidation of tyrosinase in a concentration-dependent manner. Given that 10−3 M L-tyrosine yields 10−3 M H2O2 (48⇓ , 49)⇓ , we followed tyrosinase activity in the presence of different L-tyrosine concentrations at OD 475 nm (data not shown). Tyrosinase activity is inhibited by 3 × 10−3 M L-tyrosine (▴). Inhibition can be prevented by L-Met (0–8×10−3 M) in a concentration-dependent manner, indicating that addition of L-Met prevents H2O2-induced oxidation of protein-bound methionine residues in tyrosinase (♦). D) L-Met (10×10−3 M) prevents H2O2-mediated inactivation of tyrosinase. Tyrosinase was incubated for 10 min with L-Met (10×10−3 M) together with H2O2 (0.8, 1.6, 3.2, 6.4×10−3 M), and the reaction was started on addition of L-dopa. Reaction rates were determined per minute. Result shows that excess of free L-Met protects the enzyme up to 1.6 × 10−3 M H2O2, while higher concentrations (3.2 and 6.4×10−3 M) are too high for the amount of L-Met used in the experiment. Control reaction was carried out without any addition. L-Met alone has no effect on the enzyme reaction (data not shown). All reactions were done in duplicates.
L-Methionine prevents H2O2-induced inhibition of tyrosinase
Under normal Michaelis-Menten conditions, tyrosinase is deactivated by 10−3 M L-tyrosine (22⇓ , 46⇓ , 47)⇓ . During the catalytic cycle from L-tyrosine to L-dopachrome, both H2O2 and O2 are stoichiometric byroducts (48⇓ , 49)⇓ . Hence, 10−3 M L-tyrosine yields 10−3 M H2O2. These concentrations should be sufficient to deactivate the enzyme by oxidizing Met 374 to Met-S=O, thus explaining tyrosinase suicide inhibition by its own substrate. In a first attempt to explore this hypothesis, we used different concentrations of L-tyrosine as substrate for tyrosinase. The result from this experiment confirmed our earlier finding that the enzyme is inhibited in a concentration-dependent manner (0.2–5×10−3 M) (22)⇓ (data not shown). Next, we followed the reaction in the presence of 3 × 10−3 M L-tyrosine, which would yield 3 × 10−3 M H2O2. In the presence of different L-Met concentrations (2–8×10−3 M), the inhibition can be prevented in a dose-dependent manner (Fig. 4C⇓ ). This result indicated that Met-S=O formation can be prevented on addition of L-Met. We next tested how effective L-Met could scavenge H2O2 by forming Met-S=O. To do so, we decided to follow the L-dopachrome formation of tyrosinase at 570 nm, because L-dopa is a better substrate for tyrosinase under in vitro conditions. We incubated L-Met and H2O2 in a 1:1, 5:1, and 10:1 complex for 15 min and added them to a standard reaction after 10 min. The enzyme rates were the same as in the standard reaction (0.016 min−1 mg protein−1) in the presence of 5- and 10-fold higher L-Met (0.011 min−1 mg protein−1, 0.018 min−1 mg protein−1, respectively), while the 1:1 complex was not effective (0.0031 min−1 mg protein−1) to prevent inhibition of the enzyme. These results indicate that L-Met concentrations need to exceed H2O2 concentrations in order to scavenge this ROS effectively. To support these data further, we used 10 × 10−3 M L-Met and different H2O2 concentrations (0.8–6.4×10−3 M) and incubated them with tyrosinase for 10 min. The reaction was started with addition of L-dopa. L-Met in this concentration protects the reaction in the presence of 0.8 and 1.6 × 10−3 M, whereas 3.2 × 10−3 M already exceeds the capacity at this L-Met concentration (Fig. 4D⇓ ). From these experiments, we can conclude that the protein-bound Met 374 in the active site of the enzyme is a target of H2O2-mediated oxidation. Free L-Met is an efficient H2O2 scavenger due to formation of free Met-S=O over the protein-bound Met-S=O formation, if the concentrations are sufficiently high. This result underlines once more the importance of Met 374 in the enzyme active site (29⇓ , 31⇓ 32⇓ 33)⇓ .
Inhibition of tyrosinase in melanocyte extracts by H2O2
To substantiate all above in vitro results further, we determined tyrosinase activity in native HFM and EMC extracts. HFMs yield a 1.6-fold higher activity compared to EMCs (Fig. 5A⇓ ). Our data reveal an almost complete absence of enzyme activity after H2O2-mediated oxidation of EMC extracts, supporting the in vitro results as shown with pure enzyme (Fig. 5B⇓ ).
Figure 5.
A) HFMs exhibit 1.5-fold higher tyrosinase activity compared to EMCs. Enzyme activity was determined in cell extracts from HFMs (▴; n=2) and EMCs (♦; n=2) following the L-dopachrome formation over time, as outlined in Materials and Methods. Rate of enzyme activity was calculated per milligram of protein per minute, yielding rates of 0.087 and 0.057 for HFMs and EMCs, respectively. B) Tyrosinase activity is inactivated in EMC extracts in the presence of H2O2 (2×10−3 M). Enzyme activity was determined in cell extracts from EMCs following the L-dopachrome formation over time (♦, native cell extract, n=2; ▪, oxidized cell extract, n=2) resulting in a 6.6-fold decrease after oxidation. Rate of enzyme activity was 0.066 vs. 0.010 mg−1 min−1 for native and oxidized EMC extract, respectively.
Computer modeling confirms the loss of functioning Met 374 in the active site of human and mouse tyrosinase after H2O2-mediated oxidation
To substantiate further the role of H2O2-mediated oxidation on Met 374 in the active site of the tyrosinase, we used computer simulation. The active site of the enzyme contains two copper atoms, CuA and CuB. Each is complexed by three histidines (180H, 202H, 211H and 363H, 367H, 390H, respectively) to the protein matrix provided by 2 × 2 antiparallel α-helices, α2.1, α2.2, α2.5, and α2.6, forming a 4-α-helix bundle. The loop connecting the latter two α-helices contains the sequence 374MSQVQGS380 covering not only the active site. This condition is also important for enzyme function, as shown recently by mutations (29)⇓ . The peptide oxygen atoms of 377V and 374M serve as hydrogen acceptors, and the HNδ1 groups of the imidazole rings of 180H and 367H serve as donors. In addition, another stabilizing hydrogen bond exists between the hydroxyl group of 380S and the peptide oxygen 374M. Thus, 367H and its backbone are stabilized with respect to position and orientation. The 367H is important for the function, as it orients and guides the substrate into the active site by a π-π interaction between the imidazole ring and the phenyl ring of the substrate (50⇓ , 51)⇓ . The proper orientation of 367H is guaranteed by two hydrogen bonds, one fixing the backbone via an interaction between the peptide oxygen of 367H and the HO-group of 375S and the other connecting the HNδ1 group of 367H with the peptide oxygen of 374M, which in turn builds up a hydrogen bond to 380S.
H2O2-mediated oxidation shows no significant local disturbance or sterical hindrance for the two Met-S=O diastereomers. However, several MD simulations up to a simulation time of 130 ps indicated that the structure is most likely not stable. The oxygen from 374 Met-S=O is attracted by the 367H, independent of whether the R or S form of the Met-S=O diastereomer was used in the calculations. In most cases, the HNδ1 group of 367H loses its original hydrogen bond to the peptide oxygen of 374M within a short time, forming a new hydrogen bond with the oxygen of 374 Met-S=O that remains very stable during the entire simulation time. The result is a major change in the orientation of the imidazole ring of 367H, which rotated up to 80°, while the contact distance to CuB did not change much. Thereafter, the distance between the partners of the original but disrupted hydrogen bond between the HNδ1 of 367H and the peptide oxygen of 374M fluctuated around 4 Å. Compared to H367, all other histidines in the active site kept their original position and orientation very well. Consequently, enzymatic activity is impossible, since the rotated 367H cannot orient the substrate in the appropriate way according to the proposed mechanism of tyrosinase activity (50⇓ 51⇓ 52)⇓ .
In addition, the hydrogen bond between the peptide oxygen of 377V and HNδ1 group of 180H increases to 4.4 Å and will therefore break. This condition is a result of the above-mentioned connection of the oxygen of 374 Met-S=O and the 367H, which pulls upwards the loop with 377V, while the methyl groups turn into the small cleft. Thus, 180H is affected, and therefore the CuA binding site would be destabilized as well (Fig. 6⇓ ).
Figure 6.
Homology modeling indicates severe alteration of H2O2-oxidized 374 Met-S=O on the active site of hTyrosinase. The structure of the H2O2-oxidized enzyme is considerably altered compared to the native enzyme. The important amino acids are colored and indicated. Blue, copper atoms; red, oxygen atoms; black dashes, H-bonds. MD simulation is shown at 35 ps in comparison to the start.
DISCUSSION
The in vivo identification of massive H2O2-concentrations in the gray hair shaft and the low catalase levels, as well as MSRA and MSRB activities in the entire hair follicle, introduce a new step in the understanding of human hair graying on the biochemical and molecular biological level. It opens new windows for the prevention and possible reversal of this process. The completely blunted Met-S=O repair in the graying hair follicle offers several avenues for explanation of many structural altered proteins due to oxidation of Met residues in the sequence. Clearly, this mechanism affects tyrosinase, which in turn stops melanogenesis in HFMs. The scenario of the cascade is summarized in Fig. 7⇓ . However, the high levels of H2O2 present in the hair shaft and hair follicle do not only oxidize free and bound Met residues. This ROS will also affect bound and free Cys and Trp residues as well, as shown with oxidized tyrosinase by FT-Raman spectroscopy (Fig. 7 ).
Figure 7.
H2O2 prevents Met-S=O repair in tyrosinase. In the brown hair follicle, H2O2 is generated in the micromolar range, which can activate transcription of many proteins, including catalase, tyrosinase, MSRA, and MSRB. Moreover, this ROS promotes enzyme activities in a dose-dependent manner (36, 58–60). In the presence of millimolar H2O2 concentrations, oxidation of Met, Cys, Trp, and Sec residues in protein sequences are taking place, consequently altering the tertiary structures (26, 27). These structural changes often lead to deactivation of the affected protein/enzyme. This finding has been documented for catalase, MSRA, and MSRB (23, 37, 58, 61). Low catalase levels and activities have been documented in the gray hair follicle, which in turn leads to increased H2O2 levels (43). Here, we provide evidence that tyrosinase activity is interrupted due to oxidation of Met 374 in the enzyme active site by this ROS. The resulting Met-S=O cannot be repaired, because MSRA and MSRB are also deactivated by H2O2, as evidenced by low enzyme activities in gray hair follicle extracts, or they can originate from low protein levels. The same scenario applies for catalase. Taken together, a shift in the H2O2 redox balance can significantly alter melanogenesis in the human hair follicle.
While the entire hair follicle and the hair shaft are subject to H2O2-mediated stress, it is tempting to conclude that this severely perturbed redox balance must precede melanocyte apoptosis and DNA damage, as documented earlier (4⇓ , 20)⇓ . In this context it could be possible that, besides tyrosinase and MSRA and MSRB, other proteins and peptides, including the antiapoptotic Bcl-2 protein, are targets for oxidation, which in turn could explain melanocyte apoptosis in the gray hair follicle (4)⇓ . Moreover, since low catalase levels and MSRA and MSRB are well explained by H2O2-mediated oxidation (25⇓ , 26)⇓ , the crucial open questions remain why and where these high levels of H2O2 are generated during the hair graying process. Melanogenesis in the hair follicle is certainly slowing down. Again, we use the analogy with vitiligo. H2O2-mediated oxidation has been documented for many other important regulators of pigmentation, including the proopiomelanocortins α-melanocyte-stimulating hormone and β-endorphin (53⇓ 54⇓ 55)⇓ , the prohormone convertases (55)⇓ , and the synthesis and recycling of the ubiquitous cofactor 6-tetrahydrobiopterin (56)⇓ . These regulators are required in melanocytes for intracellular L-phenylalanine turnover to L-tyrosine (57)⇓ , and many other pathways (for review see ref. 11⇓ ). It would be expected that the process of hair graying also affects these systems. Future work needs to focus on these important mechanisms.
Although no data are currently available on the HFM stem cell population, it is tempting to speculate that these cells may well also be target to oxidation. The presence of MSRA and MSRB in all hair follicle cells certainly underlines the importance of Met-S=O repair in the entire hair follicle homeostasis. Most exciting is the observation that free L-methionine can prevent Met-S=O formation of protein- and peptide-bound Met residues, saving in turn protein integrity and function, which could have great implications in the hair graying scenario in humans.
Taken together, our data provide several concordant lines of evidence, in vivo, in vitro, and in situ, in support of a severely disturbed redox homeostasis/functionality in all hair follicle cells, including the pigment-forming melanocytes as well as in the hair shaft of the anagen hair. Thus, concentration-dependent H2O2-mediated oxidation of tyrosinase in HFMs, in association with the loss of functioning Met-S=O repair, sheds a new light on the gradual slowing down of hair pigmentation as observed in the senile graying process. At this point, it is tempting to focus on the blunted methionine repair via MSRA and MSRB. Since the active site of MSRA has two crucial Met residues, it would be interesting whether L-methionine could be useful. This theory is currently under investigation. A corrected repair offers certainly an interesting target for the graying hair.
Acknowledgments
We dedicate this paper to the first author, who lost the battle against cancer during the preparation of this manuscript in February 2008. A manuscript is a fitting final word for this renowned, enthusiastic scientist. This research has been supported by the University of Bradford, by a grant to K.U.S. from Stiefel International, and by private donations. H.D. is grateful for financial support from Deutsche Forschungsgemeinschaft, Germany, and the Research Centre for Medicine and Science, Mainz, Germany. Primary human HFMs were a generous gift from Professor D. J. Tobin (University of Bradford).
References
1. ↵
Tobin, D. J., Slominski, A., Botchkarev, V., Paus, R. (1999) The fate of hair follicle melanocytes during the hair growth cycle. J. Investig. Dermatol. Symp. Proc. 4,323-332
Medline
2. ↵
Ancans, J., Tobin, D. J., Hoogduijn, M. J., Smit, N. P., Wakamatsu, K., Thody, A. J. (2001) Melanosomal pH controls rate of melanogenesis, eumelanin/phaeomelanin ration and melanosomal maturation in melanocytes and melanoma cells. Exp. Cell. Res. 268,26-35
CrossRefMedline
3. ↵
Botchkarev, V. A., Komarova, E. A., Siebenhaar, F., Botchkareva, N. V., Sharov, A. A., Komarov, P. G., Maurer, M., Gudkov, A. V., Gilchrest, B. A. (2001) p53 Involvement in the control of murine hair follicle regression. Am. J. Pathol. 158,1913-1919
Medline
4. ↵
Arck, P. C., Overall, R., Spatz, K., Liezman, C., Handjiski, B., Klapp, B. F., Birch-Machin, M. A., Peters, E. M. (2006) Towards a “free radical theory of graying”: melanocyte apoptosis in the aging human hair follicle is an indicator of oxidative stress induced tissue damage. FASEB J. 20,1567-1569
5. ↵
Lee, J. W., Harrigan, J., Opresko, P. L., Bohr, V. A. (2005) Pathways and functions of the Werner syndrome protein. Mech. Ageing Dev. 126,79-86
CrossRefMedline
6. ↵
Commo, S., Gaillard, O., Bernard, B. A. (2004) Human hair greying is linked to a specific depletion of hair follicle melanocytes affecting both the bulb and the outer root sheath. Br. J. Dermatol. 150,435-443
CrossRefMedline
7. ↵
Sarin, K. Y., Artandi, S. E. (2007) Aging, graying and loss of melanocyte stem cells. Stem Cell Rev. 3,212-217
CrossRefMedline
8. ↵
Trueb, R. M. (2005) Aging of hair. J. Cosmet. Dermatol. 4,60-72
CrossRefMedline
9. ↵
Spatz, K. R., Overall, R., Klapp, B. F., Arck, P. C., Peters, E. M. (2008) Increased melanocyte apoptosis under stress-mediator Substance P–elucidating pathways involved in stress-induced premature graying. Exp. Dermatol. 17,632
10. ↵
Slominski, A., Tobin, D. J., Shibahara, S., Wortsman, J. (2004) Melanin pigmentation in mammalian skin and its hormonal regulation. Physiol. Rev.84,1155-1228
11. ↵
Schallreuter, K. U., Bahadoran, P., Picardo, M., Slominski, A., Elassiuty, Y. E., Kemp, E. H., Giachino, C., Liu, J. B., Luiten, R. M., Lambe, T., Le Poole, I. C., Dammak, I., Onay, H., Zmijewski, M. A., Dell’Anna, M. L., Zeegers, M. P., Cornall, R. J., Paus, R., Ortonne, J. P., Westerhof, W. (2008) Vitiligo pathogenesis: autoimmune disease, genetic defect, excessive reactive oxygen species, calcium imbalance, or what else?. Exp Dermatol 17,139-140discussion 141–160
Medline
12. ↵
Slominski, A., Paus, R. (1993) Melanogenesis is coupled to murine anagen: toward new concepts for the role of melanocytes and the regulation of melanogenesis in hair growth. J. Invest. Dermatol. 101,90S-97S
CrossRefMedline
13. ↵
Chase, H. B. (1954) Growth of the hair. Physiol. Rev. 34,113-126
14. ↵
Slominski, A., Wortsman, J., Plonka, P. M., Schallreuter, K. U., Paus, R., Tobin, D. J. (2005) Hair follicle pigmentation. J. Invest. Dermatol. 124,13-21
CrossRefMedline
15. ↵
Nishimura, E. K., Granter, S. R., Fisher, D. E. (2005) Mechanisms of hair graying: incomplete melanocyte stem cell maintenance in the niche. Science307,720-724
16. ↵
Muller-Rover, S., Rossiter, H., Lindner, G., Peters, E. M., Kupper, T. S., Paus, R. (1999) Hair follicle apoptosis and Bcl-2. J. Investig. Dermatol. Symp. Proc. 4,272-277
Medline
17. ↵
Veis, D. J., Sorenson, C. M., Shutter, J. R., Korsmeyer, S. J. (1993) Bcl-2-deficient mice demonstrate fulminant lymphoid apoptosis, polycystic kidneys, and hypopigmented hair. Cell 75,229-240
CrossRefMedline
18. ↵
Nanninga, P. B., Ghanem, G. E., Lejeune, F. J., Bos, J. D., Westerhof, W. (1991) Evidence for alpha-MSH binding sites on human scalp hair follicles: preliminary results. Pigment Cell Res. 4,193-198
CrossRefMedline
19. ↵
Slominski, A., Paus, R., Plonka, P., Chakraborty, A., Maurer, M., Pruski, D., Lukiewicz, S. (1994) Melanogenesis during the anagen-catagen-telogen transformation of the murine hair cycle. J. Invest. Dermatol. 102,862-869
CrossRefMedline
20. ↵
Slominski, A., Paus, R., Plonka, P., Handjiski, B., Maurer, M., Chakraborty, A., Mihm, M. C., Jr (1996) Pharmacological disruption of hair follicle pigmentation by cyclophosphamide as a model for studying the melanocyte response to and recovery from cytotoxic drug damage in situ. J. Invest. Dermatol. 106,1203-1211
CrossRefMedline
21. ↵
Wood, J. M., Schallreuter, K. U. (1991) Studies on the reactions between human tyrosinase, superoxide anion, hydrogen peroxide and thiols. Biochim. Biophys. Acta 1074,378-385
Medline
22. ↵
Wood, J. M., Chavan, B., Hafeez, I., Schallreuter, K. U. (2004) Regulation of tyrosinase by tetrahydropteridines and H2O2. Biochem. Biophys. Res. Commun. 325,1412-1417
CrossRefMedline
23. ↵
Schallreuter, K. U., Rübsam, K., Gibbons, N. C., Maitland, D. J., Chavan, B., Zothner, C., Rokos, H., Wood, J. M. (2008) Methionine sulfoxide reductases A and B are deactivated by hydrogen peroxide (H2O2) in the epidermis of patients with vitiligo. J. Invest. Dermatol. 128,808-815
CrossRefMedline
24. ↵
Rokos, H., Wood, J. M., Hasse, S., Schallreuter, K. U. (2008) Identification of epidermal L-tryptophan and its oxidation products by in vivo FT-Raman Spectroscopy further supports oxidative stress in patients with vitiligo. J. Raman Spectrosc. 39,1214-1218
CrossRef
25. ↵
Aronoff, S. (1965) Catalase: kinetics of photooxidation. Science 150,72-73
26. ↵
Gibbons, N. C. J., Wood, J. M., Rokos, H., Schallreuter, K. U. (2006) Computer simulation of native epidermal enzyme structures in the presence and absence of hydrogen peroxide (H2O2): potential and pitfalls. J. Invest. Dermatol. 126,2576-2582
CrossRefMedline
27. ↵
Stadtman, E. R. (2006) Protein oxidation and aging. Free Radic. Res.40,1250-1258
CrossRefMedline
28. ↵
Schallreuter, K. U., Moore, J., Wood, J. M., Beazley, W. D., Gaze, D. C., Tobin, D. J., Marshall, H. S., Panske, A., Panzig, E., Hibberts, N. A. (1999) In vivo and in vitro evidence for hydrogen peroxide (H2O2) accumulation in the epidermis of patients with vitiligo and its successful removal by a UVB-activated pseudocatalase. J. Invest. Dermatol. Symp. Proc. 4,91-96
CrossRef
29. ↵
Schweikardt, T., Olivares, C., Solano, F., Jaenicke, E., Garcia-Borron, J. C., Decker, H. (2007) A three-dimensional model of mammalian tyrosinase active site accounting for loss of function mutations. Pigment Cell Res.20,394-401
Medline
30. ↵
Garcia-Borron, J. C., Solano, F. (2002) Molecular anatomy of tyrosinase and its related proteins: beyond the histidine-bound metal catalytic center.Pigment Cell Res. 15,162-173
CrossRefMedline
31. ↵
Spritz, R. A., Oh, J., Fukai, K., Holmes, S. A., Ho, L., Chitayat, D., France, T. D., Musarella, M. A., Orlow, S. J., Schnur, R. E., Weleber, R. G., Levin, A. V. (1997) Novel mutations of the tyrosinase (TYR) gene in type I oculocutaneous albinism (OCA1). Hum. Mutat. 10,171-174
CrossRefMedline
32. ↵
Spritz, R. A. (1993) Molecular genetics of oculocutaneous albinism.Semin. Dermatol. 12,167-172
Medline
33. ↵
Tripathi, R. K., Flanders, D. J., Young, T. L., Oetting, W. S., Ramaiah, A., King, R. A., Boissy, R. E., Nordlund, J. J. (1999) Microphthalmia-associated transcription factor (MITF) locus lacks linkage to human vitiligo or osteopetrosis: an evaluation. Pigment Cell Res. 12,187-192
CrossRefMedline
34. ↵
Pittelkow, M. R., Shipley, G. D. (1989) Serum-free culture of normal human melanocytes: growth kinetics and growth factor requirements. J. Cell. Physiol. 140,565-576
CrossRefMedline
35. ↵
Magerl, M., Kauser, S., Paus, R., Tobin, D. J. (2002) Simple and rapid method to isolate and culture follicular papillae from human scalp hair follicles. Exp. Dermatol. 11,381-385
CrossRefMedline
36. ↵
Schallreuter, K. U., Rübsam, K., Chavan, B., Zothner, C., Gillbro, J. M., Spencer, J. D., Wood, J. M. (2006) Functioning methionine sulfoxide reductases A and B are present in human epidermal melanocytes in the cytosol and in the nucleus. Biochem. Biophys. Res. Commun. 342,145-152
CrossRefMedline
37. ↵
Schallreuter, K. U. (2006) Functioning methionine-S-sulfoxide reductases A and B are present in human skin. J. Invest. Dermatol. 126,947-949
CrossRefMedline
38. ↵
Schallreuter, K. U., Gibbons, N. C. J., Zothner, C., Elwary, S. M., Rokos, H., Wood, J. M. (2006) Butyrylcholinesterase is present in the human epidermis and is regulated by H2O2: more evidence for oxidative stress in vitiligo.Biochem. Biophys. Res. Commun. 349,931-938
CrossRefMedline
39. ↵
Rokos, H., Moore, J., Hasse, S., Gillbro, J. M., Wood, J. M., Schallreuter, K. U. (2004) In vivo fluorescence excitation spectroscopy and in vivo FT-Raman spectroscopy in human skin: evidence of H2O2 oxidation of epidermal albumin in patients with vitiligo. J. Raman Spectrosc. 35,125-130
CrossRef
40. ↵
Krieger, E., Koraimann, G., Vriend, G. (2002) Increasing the precision of comparative models with YASARA NOVA–a self-parameterizing force field.Proteins 47,393-402
CrossRefMedline
41. ↵
Moskovitz, J., Stadtman, E. R. (2003) Selenium-deficient diet enhances protein oxidation and affects methionine sulfoxide reductase (MsrB) protein level in certain mouse tissues. Proc. Natl. Acad. Sci. U. S. A. 100,7486-7490
42. ↵
Ogawa, F., Sander, C. S., Hansel, A., Oehrl, W., Kasperczyk, H., Elsner, P., Shimizu, K., Heinemann, S. H., Thiele, J. J. (2006) The repair enzyme peptide methionine-S-sulfoxide reductase is expressed in human epidermis and upregulated by UVA radiation. J. Invest. Dermatol. 126,1128-1134
CrossRefMedline
43. ↵
Kauser, S., Westgate, G., Green, M., Tobin, D. J. (2007) Age-associated down-regulation of catalase in human scalp hair follicle melanocytes.Pigment Cell Res. 20,432
44. ↵
Schriner, S. E., Linford, N. J., Martin, G. M., Treuting, P., Ogburn, C. E., Emond, M., Coskun, P. E., Ladiges, W., Wolf, N., Van Remmen, H., Wallace, D. C., Rabinovitch, P. S. (2005) Extension of murine life span by overexpression of catalase targeted to mitochondria. Science 308,1909-1911
45. ↵
Schallreuter, K. U., Wood, J. M., Berger, J. (1991) Low catalase levels in the epidermis of patients with vitiligo. J. Invest. Dermatol. 97,1081-1085
CrossRefMedline
46. ↵
Cooksey, C. J., Garratt, P. J., Land, E. J., Ramsden, C. A., Riley, P. A. (1998) Tyrosinase kinetics: failure of the auto-activation mechanism of monohydric phenol oxidation by rapid formation of a quinomethane intermediate.Biochem. J. 333,685-691
Medline
47. ↵
Wood, J. M., Schallreuter-Wood, K. U., Lindsey, N. J., Callaghan, S., Gardner, M. L. (1995) A specific tetrahydrobiopterin binding domain on tyrosinase controls melanogenesis. Biochem. Biophys. Res. Commun.206,480-485
CrossRefMedline
48. ↵
Borovansky, J., Edge, R., Land, E. J., Navaratnam, S., Pavel, S., Ramsden, C. A., Riley, P. A., Smit, N. P. (2006) Mechanistic studies of melanogenesis: the influence of N-substitution on dopamine quinone cyclization. Pigment Cell Res. 19,170-178
CrossRefMedline
49. ↵
Land, E. J., Ramsden, C. A., Riley, P. A. (2007) The mechanism of suicide-inactivation of tyrosinase: a substrate structure investigation. Tohoku J. Exp. Med. 212,341-348
CrossRefMedline
50. ↵
Decker, H., Schweikardt, T., Nillius, D., Salzbrunn, U., Jaenicke, E., Tuczek, F. (2007) Similar enzyme activation and catalysis in hemocyanins and tyrosinases. Gene 398,183-191
CrossRefMedline
51. ↵
Decker, H., Schweikardt, T., Tuczek, F. (2006) The first crystal structure of tyrosinase: all questions answered?. Angew. Chem. Int. Ed. Engl. 45,4546-4550
CrossRefMedline
52. ↵
Decker, H., Tuczek, F. (2000) Tyrosinase/catecholoxidase activity of hemocyanins: structural basis and molecular mechanism. Trends Biochem. Sci. 25,392-397
CrossRefMedline
53. ↵
Kauser, S., Schallreuter, K. U., Thody, A. J., Gummer, C., Tobin, D. J. (2003) Regulation of human epidermal melanocyte biology by beta-endorphin. J. Invest. Dermatol. 120,1073-1080
CrossRefMedline
54. ↵
Spencer, J. D., Gibbons, N. C., Rokos, H., Peters, E. M., Wood, J. M., Schallreuter, K. U. (2007) Oxidative stress via hydrogen peroxide affects proopiomelanocortin peptides directly in the epidermis of patients with vitiligo. J. Invest. Dermatol. 127,411-420
CrossRefMedline
55. ↵
Spencer, J. D., Gibbons, N. C., Böhm, M., Schallreuter, K. U. (2008) The Ca2+-binding capacity of epidermal furin is disrupted by H2O2-mediated oxidation in vitiligo. Endocrinology 149,1638-1645
56. ↵
Schallreuter, K. U., Wood, J. M., Pittelkow, M. R., Gütlich, M., Lemke, K. R., Rödl, W., Swanson, N. N., Hitzemann, K., Ziegler, I. (1994) Regulation of melanin biosynthesis in the human epidermis by tetrahydrobiopterin.Science 263,1444-1446
57. ↵
Schallreuter, K. U., Wood, J. M. (1999) The importance of L-phenylalanine transport and its autocrine turnover to L-tyrosine for melanogenesis in human epidermal melanocytes. Biochem. Biophys. Res. Commun. 262,423-428
CrossRefMedline
58. Maresca, V., Flori, E., Briganti, S., Camera, E., Cario-Andre, M., Taieb, A., Picardo, M. (2006) UVA-induced modification of catalase charge properties in the epidermis is correlated with the skin phototype. J. Invest. Dermatol.126,182-190
CrossRefMedline
59. Hasse, S., Gibbons, N. C., Rokos, H., Marles, L. K., Schallreuter, K. U. (2004) Perturbed 6-tetrahydrobiopterin recycling via decreased dihydropteridine reductase in vitiligo: more evidence for H2O2 stress. J. Invest. Dermatol.122,307-313
CrossRefMedline
60. Schallreuter, K. U., Elwary, S. (2007) Hydrogen peroxide regulates the cholinergic signal in a concentration dependent manner. Life Sci. 80,2221-2226
CrossRefMedline
61. Wood, J. M., Schallreuter, K. U. (2006) UVA-irradiated pheomelanin alters the structure of catalase and decreases its activity in human skin. J. Invest. Dermatol. 126,13-14….