Dietary supplement increases anagen hair rate in women with telogen effluvium: results of a double-blind, placebo-controlled trial
Nadine Lengg1, Barbara Heidecker1, Burkhardt Seifert2 & Ralph M Trüeb1†
†Author for correspondence
ABSTRACT
Background: Dietary supplements are traditionally used over-the-counter products for the treatment of hair loss. Objectives: We aim to examine the effect of a specific L-cystine, medicinal yeast, pantothenic acid complex-based dietary supplement (Pantogar®) on telogen effluvium in healthy women. Methods:A randomized, double-blind, placebo-controlled study was conducted over 6 months in 30 healthy women suffering from telogen effluvium. The efficacy of the supplement was evaluated by means of digitalized epiluminescent microscopy (TrichoScan) performed before treatment and after 3 and 6 months. Additionally, global photographs were taken and evaluated by independent investigators.Results: Active compound led to a statistically significant improvement and normalization of the mean anagen hair rate within 6 months of treatment (p = 0.003), while there was no significant change in the placebo group (p = 0.85). These changes of the anagen hair rates were significantly different (p = 0.008). The appearance of hair growth in the global photographic assessment was judged better in the active compound than in the placebo group.Conclusions: This is the first study performed combining epiluminiscence microscopy with digital image analysis to demonstrate that a dietary supplement influences hair growth. The mode of action is not known, although it seems to result from an induction of anagen.
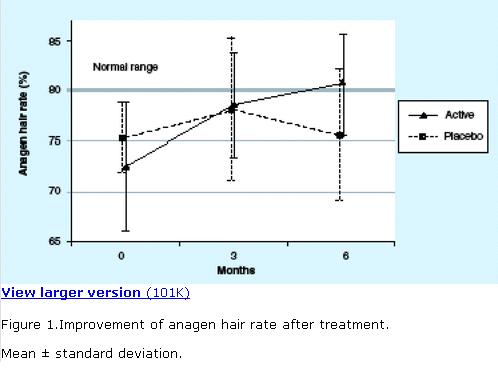
Figure 1.Improvement of anagen hair rate after treatment.
Mean ± standard deviation.
Figure 2.TrichoScan images.
Increase in the proportion of anagen hairs from (A) 65% at close-in to (B) 82% at close-out with the same hair count in the active compound group.
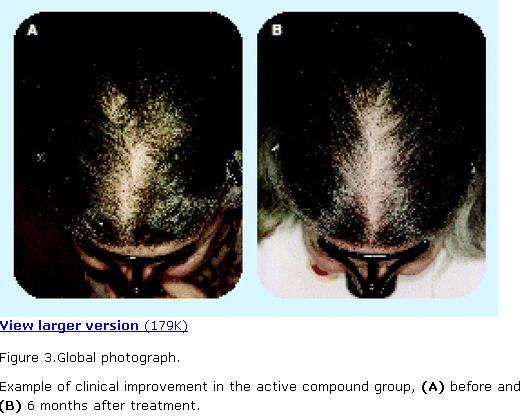
Figure 3.Global photograph.
Example of clinical improvement in the active compound group, (A) before and (B) 6 months after treatment.
Dietary supplements are traditionally used over-the-counter (OTC) products for the treatment or prevention of hair loss. Typically, they are based on a combination of L-cystine and vitamins, usually of the B-complex group, including pantothenic acid and p-aminobenzoic acid (PABA). The rationale for the use of L-cystine for the treatment of hair loss is based on the biochemistry of cystine metabolism, clinical observations in disorders of cystine metabolism and cystine deficiency and results of animal and human studies. L-cystine, a natural, aliphatic amino acid, is a constituent of keratin. Accordingly, hair contains a high proportion of L-cystine (15.9%). In trichothiodystrophy, there is a deficiency of sulfur-containing amino acids in the hair, resulting in increased brittleness. In homocystinuria, the hair is thin and hypopigmented. In HIV trichopathy, disorders of the cystine-dependent amino acid metabolism and glutathione-dependent detoxification mechanisms lead to dry, fragile hairs, hair loss and premature graying. In the 1960s, the role of L-cystine in the production of wool was investigated and it was found that enrichment of even what appeared to be a normal diet with sulfur-containing amino acids increased wool production in sheep [1–3]. When considering which dietary supplements could be used for improving hair growth in humans, L-cystine was therefore a candidate. In the early 1990s, studies on the effect of dietary supplements containing L-cystine, in combination with B-complex vitamins and medicinal yeast, a rich natural source of B-complex vitamins, were published, demonstrating improvements in the trichogram (hair pluck test), in hair swelling as a criterion for hair quality and in the tensile strength of the hair fiber [4–6].
The aim of this study was to investigate the effects of a L-cystine, medicinal yeast and pantothenic acid (CYP) complex-based dietary supplement (Pantogar®) on hair growth in healthy women with telogen effluvium using the TrichoScan.
Patients & methods
Patients & treatment
The trial was carried out as a single-center, randomized, double-blind, controlled, parallel-group study to compare the efficacy of active compound (Pantogar) with placebo in treating telogen effluvium in otherwise healthy women over a treatment duration of 6 months. Women aged 25–65 years were recruited through advertisement in the lay press.
Inclusion criteria were a history of increased hair loss, with or without clinical findings of female pattern hair loss (FPHL) Ludwig type I or II and a centroparietal telogen hair rate greater than 20%, determined by TrichoScan.
Exclusion criteria included: symptomatic diffuse alopecia (e.g., resulting from iron deficiency or thyroid gland disorder); FPHL Ludwig type III; androgenic alopecia with or without signs of virilization as result of polycystic ovaries, late onset adrenogenital syndrome or tumors of the ovaries, adrenals or pituitary gland; systemic autoimmune diseases; wasting diseases (e.g., AIDS or malignant disease); alopecia areata; inflammatory scarring or other scarring alopecias; other inflammatory conditions affecting the scalp (e.g., seborrhoeic dermatitis, psoriasis or contact dermatitis); any treatment for hair loss or participation in another clinical trial within 3 months prior to entering the study; use of drugs that may cause hair loss (e.g., anticoagulants, lipid-lowering drugs, retinoids, antiepileptics, β-blocking agents, angiotensin-converting enzyme (ACE) inhibitors, antithyroid drugs, androgens, progestagens with partial androgenic effect, aromatase inhibitors, cytokines or cytotoxic drugs) within 3 months prior to entering the study; use of sulfonamide-containing drugs (interaction with PABA); initiation or termination of hormone-replacement therapy or hormonal contraception within 6 months prior to entering the study; any type of hormone-replacement therapy or oral contraception containing a progestagen with androgenic effect (e.g., norethisterone, norgestrel, levonorgestrel, lynestrenol or tibolone); pregnancy or lactation; or known hypersensitivity to any component of Pantogar.
The study was approved by the local Ethics Committee. If the patient was suitable for the study and had given written informed consent, she was randomized into one of the two treatment arms and supplied with the active compound or placebo.
The composition of the active compound was:
•
One active compound capsule: L-cystine 20 mg, keratin 20 mg, medicinal yeast 100 mg, calcium pantothenate 60 mg, thiamine nitrate 60 mg and PABA 20 mg.
•
One placebo capsule: no active ingredient, lactose, microcrystalline cellulose and magnesium stearate.
The dosage was one capsule three-times daily with meals for the 6-month study duration.
Methods
The diagnosis of telogen effluvium was based on an increase of the telogen hair rate greater than 20% in the centroparietal scalp area, determined by the TrichoScan, and careful exclusion of other causes of hair loss. This included an in-depth history and clinical examination related to the start and duration of hair loss and its pattern. A careful personal history of diet, illness, operations and medications, including hormones, was taken. The following laboratory screening tests were performed (normal ranges): C-reactive protein (CRP; <5 mg/l), ferritin (>10 µg/l) and basal TSH (0.27–4.20 mU/l).
To determine the telogen hair rate for inclusion, the anagen hair rate, hair count, hair density and cumulative hair shaft diameter throughout the study, an area of 1.8 cm2 was defined in the centroparietal scalp using a stencil template (diameter 16 mm). In this test zone, the hair was clipped (Hairliner, Wella Germany). All clipped areas were marked with a central, single red tattoo. The tattoo was visible throughout the study. Gray or fair hairs have only limited contrast compared with the scalp, therefore, the clipped hairs within the target area were dyed with a commercially available solution (RefectoCil®, Gschwentner, Vienna, Austria). Thereafter, the colored area was cleansed with an alcoholic solution (Kodan® Spray, Schülke & Mayr, Vienna, Austria) and digital images were obtained at 20-fold (analyzed area: 0.62 cm2) magnification by means of a digital epiluminiscence microscopy (ELM) system (Fotofinder DERMA, Teachscreen Software, Bad Birnbach, Germany) while the area was still wet. This digital camera is equipped with a rigid contact lens that ensures that the images are always taken at the same distance from the scalp. Images were taken at day 0 immediately after clipping, 3 days after clipping and 3 and 6 months after the initial visit, respectively. For measurement of anagen hair rate, hair count, hair density and cumulative hair diameter, a commercially available software (TrichoScan) developed specially for this purpose was used (DatInf, Tübingen) [7].
The measures of outcome were the anagen hair rate at baseline, and after 3 and 6 months of treatment, and the hair count, hair density and cumulative hair shaft diameter at baseline and close-out (6 months). Standardized Polaroid photographs were taken for documentation of clinical outcome at baseline and close-out. For this purpose, the patients’ head was placed in a stereotactic device and Polaroid photographs were taken of the vertex and frontal areas, as described previously [8]. Assessment of the serial photographs was made independently and in a blinded manner by each of the three investigators.
Randomization of patients was performed with RANCODE version 3.6, including 50 patients in blocks of ten. Treatment 1 was verum and treatment 2 was placebo at a ratio of 1:1, with no stratification.
Statistics
Patients receiving active compound were compared with placebo at baseline (T = 0), 3 months (T = 3) and 6 months (T = 6), using the Mann-Whitney test. The statistical evaluation within one group (i.e., active compound or placebo patients) was performed with the Wilcoxon signed rank test. These procedures provide parameter-free methods with no requirements for a normal distribution of the patient population. Regression analysis was carried out to determine the influence of age, serum ferritin levels (within nomal range) and presence or not of FPHL Ludwig types I and II on the change in anagen hair rate from baseline (T = 0) to close-out (T = 6).
Results
A total of 47 patients were enrolled and 30 completed the study: 15 in the active compound group and 15 in the placebo group. In total, 17 patients withdrew from the trial due to: initiation of other treatment for hair loss (topical estradiol; one on placebo) or use of drugs that may cause hair loss (desogestrel, antiepileptic, vitamin A and β-blocker; four, all on verum), patient individually unblinding capsules (three, all on placebo), other forms of noncompliance (three on verum), intercurrent febrile illness (two, one on placebo and one on verum), intercurrent pregnancy (one on placebo), intercurrent autoimmune disease (one on verum), gastrointestinal upset (one on verum) and elevation of pancreatic enzymes (one on verum).
The age range of those who completed the study was between 38 and 61 years (mean: 51 years) in the active compound group and 25 and 61 years (mean: 46 years) in the placebo group.
In 12 patients, no thinning of hair was discernible (seven in the active compound group and five in the placebo group), 15 women showed Ludwig type I FPHL (seven in the active compound group and eight in the placebo group) and three patients had Ludwig type II FPHL (one in the active compound group and two in the placebo group).
The serum ferritin levels (inclusion criterion >10 µg/l) in the total patient population was 11–241 µg/l (mean: 62 µg/l). These levels were 11–142 µg/l (mean: 51 µg/l) in the active compound group and 13–241 µg/l (mean: 73 µg/l) in the placebo group.
The results of the anagen hair rates (as percentages) at baseline, 3 and 6 months are presented in terms of descriptive statistics in Table 1. Baseline mean anagen hair rate was similar between the active compound (73%) and placebo groups (75%). At the 3-month follow-up, both groups demonstrated improved anagen hair rates (78% in both groups) that was satistically not significant. At 6 months, the benefit from active treatment showed an additional increment of the mean anagen hair rate to 81%, in contrast with placebo, which practically fell back to baseline. The most remarkable finding was that the active compound group reached a physiological range of anagen hair rate greater than 80%, while the placebo group did not (Figure 1). Statistical analysis of the change in the mean anagen hair rate in the active compound group within the 6 months of treatment showed a statistically significant improvement (p = 0.003), while there was no significant change in the placebo group (p = 0.85). Analysis of the change of the mean anagen hair rate within the active compound group compared with that of the placebo group in the 6 months of treatment was also statistically significant (p = 0.008).
The hair count, hair density and cumulative hair shaved diameter were not significantly different from the baseline values in either group (Table 2).
The appearance of hair growth in the global photographic assessment was judged better in the active compound than in the placebo group (Table 3).
Finally, regression analysis did not show any effect of age, presence of visible hair thinning in the vertex areas (FPHL) and serum ferritin levels above the lower limit of normal (10 µg/l) on changes in anagen hair rate.
The active compound was generally well tolerated. Four patients reported gastrointestinal symptoms, four complained of weight gain and one had transient elevation of serum pancreatic enzymes, which was probably not related to the intake of active compound.
Discussion
Telogen effluvium in apparently healthy women was originally considered a distinct entity [9]. Since the recognition of FPHL in women by Ludwig [10], it seems that a majority of cases have been attributed to this diagnosis. The differential diagnosis includes symptomatic hair loss resulting from hormonal disorders, malnutrition, preceding or concomitant internal disease or adverse drug reactions [11]. In an estimated third of women with persistent telogen effluvium, no precipitating cause can be found. On the one hand, it is believed that complex etiologies underlie this type of hair loss, in which there is not only one, but several factors interacting to cause the hair loss. On the other hand, ‘diffuse androgen-dependent alopecia’ was proposed as an alternative explanation, on the assumption that it is also androgen-induced and therefore FPHL [12]. However, controversy has arisen with respect to the role of androgens in FPHL (since FPHL may occur before the onset of puberty), has been described in individuals with hypogonadism and does not respond to treatment with cyproterone acetate or finasteride in the absence of pathologically elevated androgen levels [13–17]. Finally, diffuse cyclic hair loss in women, first described by Guy and Edmundson [18], has recently been redefined as a distinct entity on the basis of histological studies and re-named ‘chronic telogen effluvium’ (CTE) [19]. This type of hair loss affects otherwise healthy women complaining of persistent diffuse hair loss [20]. The authors attribute the disorder to synchronization phenomena of hair cycling. It actually represents exaggerated hair shedding or teloptosis, rather than regressive alopecia that is characteristic of FPHL or senescent alopecia. Accordingly, FPHL eventually presents with visible thinning of the hair over the vertex area, and senescent alopecia as age-dependent diffuse thinning of hair, as a result of progressive shortening of the anagen phase of the hair cycle. By contrast, CTE essentially does not cause thinning of the vertex area. Although FPHL is not primarily due to synchronization of hair cycling, partial synchronization phenomena tend to complicate its course, particularly on a seasonal basis in autumn [21]. It has correctly been noted that the combination of FPHL with CTE represents a potential complication for clinical trials, with drugs such as minoxidil developed specifically for FPHL [22].
For the treatment of FPHL, minoxidil has proven efficacy, while the management of CTE and the role of dietary supplements for this indication have remained controversial. Previous accounts of a benefit from the active compound Pantogar on hair growth have been based on patient questionnaires and trichogram examinations [4–6]. Direct questioning of users is popular in the assessment of hair therapeutics; however, everyday practice shows that it is not easy to put subjective complaints of hair loss into objective terms, or to evaluate treatment efficacy, particularly in women [23]. Office-based techniques for evaluating hair loss, such as the trichogram and global photographic assessment, are of limited use owing to poor reproducibility and are useful only in a minority of patients who show clinically discernible improvement of hair growth, respectively. Thus, a simple to perform, noninvasive, reproducible method for objective measurement of hair growth parameters was required. For this purpose, the TrichoScan was developed, which combines ELM with digital imaging [7]. Relevant hair growth parameters measured with this technique are: anagen hair rate, hair count, hair density and cumulative hair shaft diameter.
This is the first study performed with the TrichoScan to demonstrate that a CYP complex-based dietary supplement (Pantogar) influences hair growth. After 6 months of treatment, the active compound group showed a statistical significant improvement in anagen hair rate, while the placebo group did not. Although there was a large number of drop-outs from the study (over a third of those enrolled), a breakdown of withdrawal criteria in relation to placebo or active compound groups, following unblinding after termination of the study, did not suggest that exclusion of these subjects should have biased the results. Improvement of the anagen count has previously also been shown for treatment of hair loss in women with another combination product with L-cystine using the phototrichogram [24]. The hair count, hair density and cumulative hair shaft diameter did not show significant changes from the baseline values. Nevertheless, the increase in anagen hair rate was reflected in clinical outcome, since the appearance of hair in the global photographic assessment was also judged better in the active compound group (Figure 2 & 3) compared with placebo (Table 3). A conceivable explanation for this observation would be the proportionate increase in the number of terminal hairs in anagen, that is, actively growing terminal hairs. This is not detected by the hair count, hair density, and cumulative hair shaft diameter, since these mainly reflect a gain of hair mass through vellus-to-terminal hair transformation. It is imaginable that these observations would also exclude newly acquired hypertrichosis as undesired effect of the CYP complex-based compound (in contrast to minoxidil) since, on the body, vellus hairs would only be driven into anagen. The mechanism of action is not known, although these data suggest that the CYP complex-based active compound (Pantogar) has a therapeutic effect owing to an induction of anagen. A similar effect has been shown for topical melatonin, which is known to have physiological effects on the hair cycle [25].
Since regressive alopecias, such as FPHL and senescent alopecia, are due to progressive shortening of the anagen phase and miniaturization of the hair follicle, it is apparent that they benefit from treatment with an agent with impact on hair count, hair density and cumulative hair shaft diameter, such as minoxidil. Nevertheless, synchronization phenomena tend to complicate the course of regressive alopecias since, with a shortened anagen phase, synchronization will tend to be more marked. In this case, it is imaginable that adding a CYP complex-based dietary supplement to the treatment regimen may be beneficial. Moreover, it has been shown in whole-hair follicle cultures that minoxidil not only increases the incorporation of thymidine (as a marker of cell division), but also leads to an increased uptake of cysteine by the hair follicle [26]. Consistent with this, regression analysis showed that age (in view of senescent alopecia) and presence of hair thinning at the vertex (in view of FPHL) did not influence changes in anagen hair rate.
Finally, serum ferritin levels within the normal range above 10 µg/l did not influence changes in anagen hair rate.
Conclusion
In summary, this is the first randomized, double-blind, placebo-controlled study performed with the TrichoScan to demonstrate that a CYP complex-based dietary supplement (Pantogar) leads to an increase of anagen hair rate and an improvement of the overall photographic appearance within 6 months in otherwise healthy women with telogen effluvium. Due to its action on synchronization phenomena of hair cycling, indications for CYP complex-based dietary supplementation (Pantogar) would thus include postpartum effluvium, seasonal effluvium and diffuse cyclic hair loss in women or CTE. The effects were observed irrespective of patient’s age, presence of FPHL and serum ferritin levels within the normal range above 10 µg/l. Therefore, it is conceivable that CYP complex-based dietary supplementation may be useful in addition to minoxidil therapy in the treatment of FPHL, whenever there is partial synchronization in telogen. Iron supplementation therapy seems to be of less relevance in the treatment of diffuse hair loss in healthy women with normal serum ferritin levels, as previously also challenged by other authors [27,28].
Table 1.Results of anagen hair count (%).
Table 2.Results of hair count, hair density and cumulative hair shaft diameter.
Table 3.Assessment of global photographs.
Highlights
•Dietary supplements, such as L-cystine, medicinal yeast and pantothenic acid (CYP complex) are traditionally used over-the-counter products for the treatment of hair loss.
•This is the first randomized, double-blind, placebo-controlled study performed combining epiluminiscence microscopy with digital image analysis (TrichoScan) to demonstrate that a CYP complex-based dietary supplement influences hair growth.
•The mode of action remains unknown, although it seems to result from induction of anagen.
•Active coumpound led to a statistically significant improvement and normalization of the mean anagen hair rate within 6 months of treatment in otherwise healthy women with telogen effluvium.
Disclaimer
This study represents the Inaugural Dissertation of Nadine Lengg, University of Zurich, Switzerland. The results were presented by Ralph M Trüeb on the occasion of the 11th Annual Meeting of the European Hair Research Society in Zurich, 7th–9th July, 2005.
Acknowledgement
The authors acknowledge Rolf Hoffmann, Freiburg i.B., Germany, for assistance in analyzing the TrichoScan images. This study was performed with financial support from Merz Pharmaceuticals GmbH, Germany.
Bibliography
1 . Gillespie JM, Reis PJ: Dietary regulated biosynthesis of high-sulfur wool proteins. Biochem. J.98,669–677 (1966). [Medline]
2 . Reis PJ, Tunks DA, Sharry LF: Plasma amino acid patterns in sheep receiving abomasal infusions of methionine and cystine. Aust. J. Biol. Sci.26,635–644 (1973). [Medline]
3 . Frenkel MJ, Gillepsie JM, Reis PJ: Factors influencing the biosynthesis of the tyrosine-rich proteins of wool. Aust. J. Biol. Sci.27,31–38 (1974). [Medline]
4 . Petri H, Perchalla P, Tronnier H: Die wirksamkeit einer medikamentösen therapie bei haarstrukturschäden und diffusen effluvien – vergleichende doppelblindstudie. Schweiz Rundsch Med Prax79,1457–1462 (1990). [Medline]
5 . Budde J, Tronnier H, Rahlfs VW, Frei-Kleiner S: Systemische therapie von diffusem effluvium und haarstrukturschäden. Hautarzt44,380–384 (1993). [Medline]
6 . Ahrens J: Systemische behandlung des diffusen haarausfalls. Therapiewoche Schweiz10,551–554 (1994).
7 . Hoffmann R: TrichoScan: combining epiluminiscence microscopy with digital image analysis for the measurement of hair growth in vivo. Eur. J. Dermatol.11,362–368 (2001). [Medline]
8 . Trüeb RM, Itin P: Schweizerische arbeitsgruppe für trichologie: fotografische dokumentation der wirksamkeit von 1 mg oralem finasterid in der behandlung der androgenetischen alopezie des mannes im praxisalltag. Schweiz Rundsch Med Prax90,2087–2093 (2001).
9 . Sulzberger MB, Witten VH, Kopf AW: Diffuse alopecia in women. Its unexplained apparent increase in incidence. Arch. Dermatol.81,556–560 (1960). [Medline]
10 . Ludwig E: Classification of the types of androgenetic alopecia (common baldness) occurring in the female sex. Br. J. Dermatol.97,247–254 (1977). [CrossRef] [Medline]
11 . Trüeb RM: Diffuser haarausfall bei frauen. Therapeutische Umschau.59,217–222 (2002). [CrossRef] [Medline]
12 . Rushton DH, Ramsay ID, James KC et al.: Biochemical and trichological characterization of diffuse alopecia in women. Br. J. Dermatol.123,187–197 (1990). [CrossRef] [Medline]
13 . Birch MP, Messenger JF, Messenger AG: Hair density, hair diameter and the prevalence of female pattern hair loss. Br. J. Dermatol.144,297–304 (2001). [CrossRef] [Medline]
14 . Norwood OT: Incidence of female androgenetic alopecia (female pattern alopecia). Dermatol. Surg.27,53–54 (2001). [CrossRef] [Medline]
15 . Orme S, Cullen DR, Messenger AG: Diffuse female hair loss: are androgens necessary? Br. J. Dermatol.141,521–523 (1999). [CrossRef] [Medline]
16 . Vexiau P, Chaspoux C, Boudou P et al.: Effects of minoxidil 2% vs. cyproterone acetate treatment on female androgenetic alopecia: a controlled, 12-month randomized trial. Br. J. Dermatol.146,992–999 (2002). [CrossRef] [Medline]
17 . Price VH, Roberts JL, Hordinsky M et al.: Lack of efficacy of finasteride in postmenopausal women with androgenetic alopecia. J. Am. Acad. Dermatol.43,768–776 (2000). [CrossRef] [Medline]
18 . Guy WB, Edmundson WF: Diffuse cyclic hair loss in women. Arch. Dermatol.81,205–27 (1960). [Medline]
19 . Whiting DA: Chronic telogen effluvium: increased scalp hair shedding in middle-aged women. J. Am. Acad. Dermatol.35,899–906 (1996). [CrossRef] [Medline]
20 . Trüeb RM: Das idiopathische chronische telogen effluvium der frau. Hautarzt51,899–905 (2000). [CrossRef] [Medline]
21 . Randall VA, Ebling FJG: Seasonal changes in human hair growth. Br. J. Dermatol.124,146–151 (1991). [CrossRef] [Medline]
22 . Rand S: Chronic telogen effluvium: potential complication for clinical trials in female androgenetic alopecia? J. Am. Acad. Dermatol.37,1021 (1997). [CrossRef] [Medline]
23 . Guarrera M, Semino MT, Rebora A: Quantitating hair loss in women: a critical approach. Dermatology194,12–16 (1997). [CrossRef] [Medline]
24 . Gehring W, Gloor M: Das phototrichogramm als verfahren zur beurteilung haarwachstumsfördernder präparate am beispiel einer kombination von hirsefruchtextrakt, l-cystin und calciumpantothenat. Zeitschrift für Hautkrankheiten75,419–423 (2000).
25 . Fisher TW, Burmeister G, Schmidt HW et al.: Melatonin increases anagen hair rate in women with androgenetic alopecia or diffuse alopecia: results of a pilot randomized controlled trial. Br. J. Dermatol.150,341–345 (2004). [CrossRef] [Medline]
26 . Buhl AE, Waldon DJ, Kawabe TT et al.: Minoxidil stimulates mouse vibrissae follicles in organ culture. J. Invest. Dermatol.92,315–320 (1989). [CrossRef] [Medline]
27 . Aydingoz IE, Ferhanoglu B, Guney O: Does tissue iron status have a role in female alopecia? J. Eur. Acad. Dermatol. Venereol.13,65–67 (1999). [CrossRef] [Medline]
28 . Sinclair R: There is no clear association between low serum ferritin and chronic diffuse telogen hair loss. Br. J. Dermatol.147,982–984 (2002). [CrossRef] [Medline]
Affiliations
Nadine Lengg
1Department of Dermatology, University Hospital of Zurich, Gloriastr. 31, 8091 Zurich, Switzerland. ralph.trueeb@usz.ch
Barbara Heidecker
1Department of Dermatology, University Hospital of Zurich, Gloriastr. 31, 8091 Zurich, Switzerland. ralph.trueeb@usz.ch
Burkhardt Seifert
2Department of Biostatistics, Institute for Social and Preventive Medicine, University of Zürich, Switzerland.
Ralph M Trüeb
1Department of Dermatology, University Hospital of Zurich, Gloriastr. 31, 8091 Zurich, Switzerland. ralph.trueeb@usz.ch
Cited by
Gloria Garnacho-Saucedo, Rafael Salido Vallejo, Maria Ángeles Álvarez López, Jose Carlos Moreno Giménez. (2012) Estudio y tratamiento de los efluvios. Piel 27:9, 511-520
Online publication date: 1-Nov-2012.
CrossRef
Ralph M. Trüeb. (2010) Systematic approach to hair loss in women. JDDG: Journal der Deutschen Dermatologischen Gesellschaft 8:4, 284-297
Online publication date: 1-Apr-2010.
CrossRef
C. Durusoy, Y. Ozenli, A. Adiguzel, I. Y. Budakoglu, O. Tugal, S. Arikan, A. Uslu, A. T. Gulec. (2009) The role of psychological factors and serum zinc, folate and vitamin B 12 levels in the aetiology of trichodynia: a case-control study. Clinical and Experimental Dermatology 34:7, 789-792
Online publication date: 1-Oct-2009.
CrossRef